Pharmaceutical Product Registration in Malaysia – Overview
The Drug Control Authority (DCA) is responsible for drug registration process in malaysia. The registration is done through the web-based QUEST 3+ online system.
Different Regulatory requirements apply to each category of medicinal products, and adherence to these requirements is essential for Malaysia pharmaceutical products registration and approval.
The National Pharmaceutical Regulatory Agency (NPRA) conducts scientific and technical reviews, inspections, and surveillance activities for drug registration process in Malaysia.
With a primary focus on drug registration in Malaysia, the NPRA meticulously conducts multifaceted reviews, inspections, and vigilant surveillance activities.
The Malaysia Drug Registration and Approval process involves comprehensive assessments form the cornerstone of the Regulatory process, ensuring that all pharmaceutical products seeking entry into the Malaysian market meet stringent quality standards and Regulatory prerequisites.
The NPRA's commitment to drug registration in Malaysia underscores its dedication to safeguarding public health by meticulously evaluating each product's compliance, thereby fostering confidence in the pharmaceutical landscape.
As an authoritative body, NPRA's continuous efforts in drug registration in Malaysia significantly contribute to maintaining the integrity of the country's pharmaceutical market and ensuring the well-being of its populace.
The DCA oversees the registration of pharmaceutical products and cosmetics, licensing of premises, and monitoring the quality of registered products and Adverse Drug Reactions (ADR). The NPRA is one of the participating authorities in the Pharmaceutical Inspection Co-operation Scheme (PIC/S).
Need help with Malaysia pharmaceutical products registration and approval? Contact our expert today!
Medicinal Product Classification in Malaysia
Medicinal products are classified into the following categories:
- New Drug Product (NDP)
- Biologics
- Generics [Prescription Medicine & Non-prescription Medicine, Over the Counter (OTC)]
- Health and Food Supplements
- Natural Products
- Veterinary Products
- Medicinal Gas
Drug Product Registration in Malaysia
It is important to determine the product category for distinct pharma product registration activities, as it directly impacts the drug development & approval process in Malaysia and Pharmaceutical Regulatory Affairs in Malaysia. Understanding the classification ensures that the product complies with the necessary Regulatory requirements, including Malaysia Drug Registration and approval process for both generic drug registration in Malaysia and new drug registration in Malaysia.
The pharmaceutical product applicant is known as the Product Registration Holder (PRH). A PRH must be a locally incorporated company, corporate, or legal entity with a permanent address and should be registered with the Companies Commission of Malaysia.
For any foreign medicinal product manufacturer, it is mandatory to have a local entity or local presence in Malaysia for pharmaceutical product registration in Malaysia with the NPRA.
The appointed agent is responsible for all matters relating to product quality, safety, and efficacy for pharmaceutical product registration in Malaysia.
The drug company must follow the NPRA product registration guideline and the Association of Southeast Asian Nations (ASEAN) Common Technical Documents (ACTD) to be certified as compliant for the registration of pharmaceutical products in Malaysia.
The Malaysia drug Registration and approval process must be followed for the registration of pharmaceutical products in Malaysia. The DCA is also responsible for reporting Adverse Drug Reactions (ADRs). Any reports of adverse reactions associated with the use of registered products in Malaysia must be reported to the DCA within the stipulated timelines.
The DCA should be advised of any significant safety issues. The NPRA product registration is conducted through the web-based QUEST 3+ Online System.
Get help with Malaysia pharmaceutical products registration and approval from Freyr today!
ASEAN Common Technical Document (ACTD)
|
Parameter |
Certificate/Document required |
|
Quality |
GMP certificate, Product testing data specification, Microbial limit test, and other required quality documents, if required |
|
Safety |
Preclinical & clinical analysis data, product information - warning labels/precautions/drug interactions/adverse effects and other required safety documents, if required |
|
Efficacy |
Clinical trial data - Phase 2 & 3, bioequivalence studies, and other required efficacy documents, if required |
Regulatory Pathway for Pharmaceutical Product Registration in Malaysia
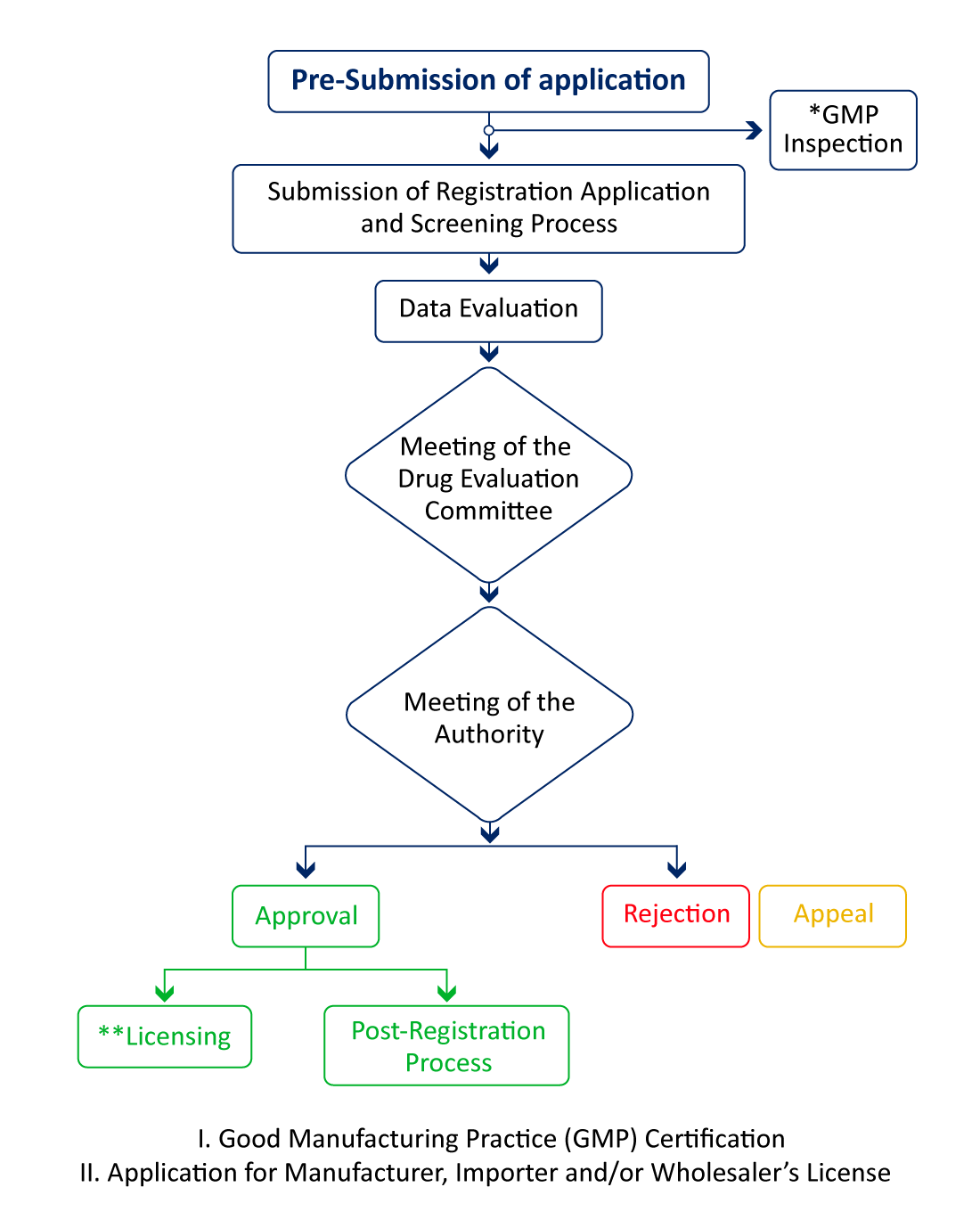
If the manufacturing site is in any of these reference countries - USA, Canada, UK, Europe, Japan, Australia, and Singapore, an NPRA site visit is not required unless they have a certain reason for inspection, and the review process can be expedited for quicker pharma market access.
API manufacturers are required to have a WHO-GMP certification to register their finished products. The WHO-GMP certification is mandatory to get the site and product registrations completed.
Product Maintenance & Compliance
|
Sr. No |
Application |
Validity |
|
01 |
Validity for Registration Certificate |
Five (05) years from the date of registration |
|
02 |
Renewal of Registration certificate |
The renewal application should be submitted six (06) months before the expiration of the existing registration certificate |
|
03 |
Post-approval Changes (Variations) |
Any change of registered medicine should be sent to the NPRA with relevant documents, on a case-by-case basis |
Pharmaceutical Regulatory Affairs in Malaysia - Freyr Expertise
- Drug development & approval process in Malaysia.
- Authorized Local Representation.
- Support for generic drug registration in Malaysia and new drug registration in Malaysia.
- Product Classification & Drug Registration in Malaysia.
- Regulatory Affairs Consulting and Pharma Market Access.
- End-to-end Pharmaceutical Product Registration in Malaysia/Drug Product Registration Process in Malaysia.
- Gap Analysis, Pharmaceutical Dossier Preparation, and Submission to the NPRA.
- Preclinical Services (Generation of Scientific Reports for Regulatory Submissions).
- Import License Application.
- NPRA Drug Registration.
- Change of Product Registration Holder.
- Ad-hoc Regulatory Affairs Consultation.
Do you want to streamline your Regulatory pathway to Malaysia pharmaceutical product registration and approval? Contact our Freyr experts today!